Written by Dr. Skye Zeller, M.D.
Today I am side tracking from my usual airway blog to post on the newest vaccines that have been FDA EUA approved to help in the fight against COVID-19. If you haven’t read out “about us” page, I spent 7 years earning my PhD from Yale in microbiology before I left research to begin my dental journey. My thesis was focused on developing RNA based therapeutics to prevent HIV infection. These two new vaccines are RNA based vaccines and with the background knowledge I had, I felt that it was important to chime in on some misinformation that I have been listening to. All the data that I have summarized here is from the actual FDA submission from both Moderna and Pfizer as well as through the preclinical studies that I have read from both companies. Again, none of this is hearsay, none of this is conjecture, all of this is from data. I have no conflicts of interest as I have nothing invested in either company. I just hope that reading through this write-up can help some people make their own risk/benefit analysis and decide if they feel comfortable getting the vaccine rather than being at risk of serious COVID infection. I will NOT give anyone advice on what they personally should or should not do, but I will say that I am signed up to get the Moderna vaccine on Tuesday!
How does this vaccine work?
In order to understand this we must first understand the way the virus works, the way the immune system works, and the way that this vaccine was developed:
How does SARS-CoV2 infect my cells?
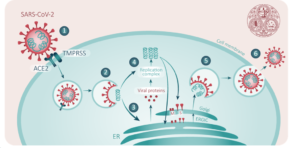
The virus that causes COVID19 is a single stranded RNA virus. In order for the virus to enter your cells, the “spike” protein acts like a “key” binding with the ACE2 protein (or the lock) on the surface of the cells. This interaction causes the virus to be engulfed (endocytosed) into the cells. The virus undergoes a series of conformational changes (moves around a bit) and the viral RNA (or the instructions to make more of the virus) is dumped into your cells. Now that the instructions (RNA) are in the cell, the cell starts to make virus proteins. From these virus proteins, more copies of the virus RNA are made. Now your cell has turned into a mini virus production factory cranking out copies of viral RNA and viral proteins that can assemble to form new viral particles that are released from the cell and begin to circulate through the body finding more cells that they can infect. Importantly for them to infect another cell, this cell must express the “lock” or ACE2 protein on the surface. Different cells express different proteins on the surface and the cells that express ACE2 on the surface at the highest levels are heart, lung, kidney, and GI cells.
The idea with this vaccine is to neutralize or block the “spike protein” (think key) from binding to the “lock” (ACE2) and preventing it from being able to enter and infect the cells.
How does the immune system work to help block the spike protein?
o In a super simplified version of how the immune system works we need to understand a few key points. First, the immune system is made to recognize foreign objects and attack them. It does this both through antibody production (b cells) and by killing off infected cells (CD8 T cells). Once our immune system recognizes a foreign object, it takes a little time to get the troops up and running, to prime the system and to have a reserve of B and T cells that can fight off the infection. Once that happens, then these cells are primed and ready to produce antibodies and attack once the virus is encountered again.
o Second, an antibody is basically a giant protein that sticks to its target with precision. For example, if your body generates antibodies against protein A, those antibodies will not bind to protein B. A “neutralizing” antibody is an antibody that will block the function of its target when it is bound by the antibody. Going back to the lock and key analogy- if a neutralizing antibody bind to the “key” then the “key” can no longer bind to and open the “lock”.
o People who have been infected with COVID19 develop neutralizing antibodies. This is the basis behind using convalescent serum as a treatment for severe covid infection. Scientists who looked at the most neutralizing anitbodies from this serum were able to identify antibodies that bound to certain parts of the spike protein as having the highest neutralizing potential. This is great for vaccine development because spike protein must stay mostly conserved (without major mutations) or the virus cannot bind and infect our cells.
How does the vaccine work?
o Many vaccines are either attenuated (weakened) versions of the virus or heat killed versions of the virus. The reasons this vaccine is getting so much attention is that it is a new approach to generating an immune response. This vaccine uses a lipid nanoparticle to encompass a messanger RNA that encodes a highly antigenic (immune response inducing) portion of the viral spike (again think of RNA like instructions for the cell to make proteins). So basically it’s a little ball of instructions being delivered into your cells that will tell your cells to make only the one part of the spike protein. This protein is then displayed on the surface of your cells, the immune system begins to see this protein as foreign and starts to prime the immune system to respond if it sees this protein again.
o The next time that your body sees this protein (for example you get exposed to COVID19) then your cells are ready to go and begin secreting neutralizing antibody to block the spike protein from binding to the ACE2 receptor on the surface of the cells.
o IMPORTANT: This technology has been around for over a decade and is not a brand new idea. This technology was not ‘warp speed” or “rushed” but has been in development for use against other diseases and targets for decades. Lipid nanoparticles have been used in the treatment of liver cancer and in amyloidosis in humans since 2013. In fact, the first RNA based drug was FDA approved in 1998 for the treatment of CMV Retinitis. What is novel is that this is the first time an RNA based vaccine has been used in clinical trials.
What side effects can I expect after getting the vaccine?
I want to break down side effects by age group (under 55 y./o and over 65 y/o) and into probable side effects and things that showed a numerical difference between the 2 groups but no statistical difference.
Under 55 y/o:
Over 55
Serious Adverse events with numerical imbalances but no significant differences:
NO serious adverse events were noted to be statistically significant, but there are overall a low number of SAEs in the study. As more patients get vaccinated, the FDA will be monitoring any additional cases of Bell’s palsy.
I heard that the vaccine can alter my DNA?
This is incorrect. An important thing to understand is that humans lack the enzyme to turn RNA into DNA. This enzyme is encoded by viruses such as HIV that are in the retrovirus family (retro because instead of flowing from DNA to RNA they can make RNA into DNA). RNA cannot incorporate into DNA and because we cannot make RNA into DNA in our cells, we do not have the potential for the vaccine RNA to incorporate in our DNA.
Can I still get COVID if I get the vaccine?
Because of the way the study was designed, the only data we have is on symptomatic COVID infection. This means that after vaccination, a participant was only tested for COVID if they developed symptoms that warranted being tested. These symptoms included fever, cough, shortness of breath, chills, muscle pain, loss of taste or smell, sore throat, diarrhea, vomiting. Because participants were not routinely tested, we have no data on the rate of non-symptomatic infection. This means that there is a possibility that YES you can still get COVID even if you get the vaccine. But that the course of infection is more likely to be asymptomatic.
Pre-clinical studies in monkeys (rhesus macaques) showed that when vaccinated and then challenged with SARS-CoV2 the researchers were able to recover virus from the nose (nasal and nasopharyngeal swabs) but not from the lungs (bronchoalveolar lavage fluid). Is suggests that infection can still occur but at limited severity.
The data does show that when vaccinated there were only 8 symptomatic COVID cases in the vaccine group vs. 162 symptomatic COVID cases in the control group. Again there is no data on asymptomatic cases as this did not trigger testing for COVID.
Severe COVID cases were numerically imbalanced between vaccine and placebo groups with 1 vaccine recipient and 3 placebo recipients having COVID cases classified as severe. There were not enough cases of severe COVID to make any conclusion on differences in occurrence of severe disease. However, based on the greatly reduced levels of symptomatic COVID infection (95% efficacy), many have made the assumption that vaccination will prevent from severe COVID infection as well.
Do I still need to keep up with social distancing and mask wearing?
Yes. Because we have no data on vaccine preventing asymptomatic infection, it is important that you do not become a source of infection for other individuals who have not yet been vaccinated. We do not know if people who have been vaccinated will be able to transmit the virus at this point. So social distancing and mask wearing remain important to prevent the spread of the virus while we await vaccination levels that can stop the spread of covid (estimates are currently around 75% of the world population).
If I already had COVID should I get the vaccine?
Given the lack of good data on this point, I would personally not make a recommendation either way. In the study by Pfizer, 3% of participants had shown evidence of a prior infection (antibodies against the virus at initial blood draw). Of those 1,320 individuals who had been previously infected with COVID, only 19 cases of COVID were reconfirmed. This is a 1.4% infection rate and is similar to the infection rate of the overall group suggesting that natural long term immunity to this virus may be hard to achieve. However, we do know that neutralizing antibodies remain in previously infected individuals for up to 3 months and that reinfection in those 3 months is rare. Therefore, recommendations have been made to delay vaccination of people with prior infection for 3 months after infection.
I have an underlying condition- is it safe for me to get the vaccine?
In this study certain underlying comorbidities were included. Of note, this included 120 individuals with HIV infection, obesity (35% of participants), diabetes (8.4%), pulmonary disease (7.8%). Other underlying conditions included liver disease, congestive heart failure and hypertension. Among subgroup analyses there were no differences in safety or efficacy across groups with comorbidities and groups without. Based on the risk/benefit profile, those with underlying conditions face the highest risk of severe covid infection and therefore have a high benefit from receiving the vaccine with a minimal risk of adverse reaction from the vaccine itself.
What are some major pitfalls of the studies at this point?
The major pitfalls as I see them are really just gaps in knowledge because we have limited data in terms of size of groups and time from immunization. The FDA is requiring that any adverse event be reported to the vaccine monitoring database within 15 days of its occurrence and this database is publicly available. Therefore, in the next few weeks we should see if any trends occur in the side effect profile of the Pfizer vaccine.
What are the key differences between Pfizer and Moderna vaccines?
Both of these vaccines have high efficacy (overall analysis of 94+% efficacious in preventing symptomatic COVID infection). The make-up of the two vaccines is similar (lipid nanoparticle encompassing mRNA encoding the spike protein). And the adverse reactions to the vaccines were also very similar in terms of most frequently reported symptoms. I have summarized the differences in the table below.

Both vaccines have been given an EUA from the FDA.
Key differences are dosing, time between initial and boosted and storage temperature of the vaccines.
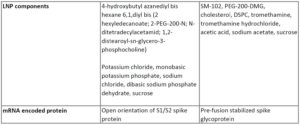
Both vaccines are lipid nanoparticles that encompass mRNAs that encode for the spike protein.
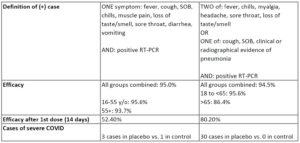 The studies were both designed to look at reduction of symptomatic COVID-19 cases. An individual was only marked as having COVID if they met the criteria for a positive case. Given those parameters, the overall efficacy for both vaccines was ~95%. It does seem that moderna has a lower efficacy in the >65 crew. But it does show better efficacy after a single dose (starting 2 weeks after dose 1).
The studies were both designed to look at reduction of symptomatic COVID-19 cases. An individual was only marked as having COVID if they met the criteria for a positive case. Given those parameters, the overall efficacy for both vaccines was ~95%. It does seem that moderna has a lower efficacy in the >65 crew. But it does show better efficacy after a single dose (starting 2 weeks after dose 1).
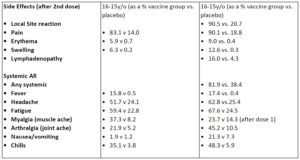
Major side effects seen in the younger cohort included localized reactions ()pain, swelling, redness) as well as generalized symptoms (headache, fatigue). these were worse after the second dose and the numbers I show here are symptoms after dose 2.
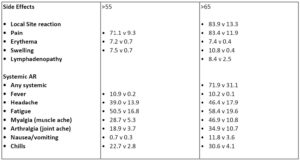
Side effects were milder for those in the older cohort for both vaccines. Overall it seems that pfizer has a lower percentage of participants reporting symptoms than moderna.

Serious Adverse events- Of note, none of these are thought to be due to the vaccine. If I took a group of 30-40,000 people we should see these same numbers of Bells palsy in that cohort. However, there is a possibility that the cases of facial swelling in individuals who report use of dermal fillers is linked to the vaccine. My guess is either an interaction between the lipid particles and what they use for fillers- this is strictly my best guess- there is NO data on this yet.
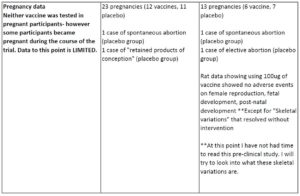
The ACOG is recommending that pregnant women be given the option to get vaccinated. There is, however, no clinical data in pregnant women at this point.
So, there you have it. All 100+ pages of FDA data summarized for you. I hope this helps you to make the right choice for you and your family when it is your turn to get the vaccine. As I said above, I am signed up to get it in less than 72 hours and I will be honest about any reactions that I have. I trust the scientists that have worked for decades on this technology and I trust the FDA. I also trust that I have read and educated myself with all the data that is available. If you have any questions, I am happy to answer whatever I can! Stay safe!!!
Skye Zeller, M.S.
StayWell Health Center